Steel and Concrete: an «almost» perfect combination
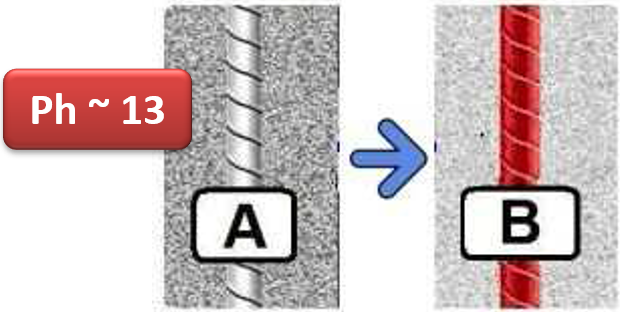
In reinforced concrete the surface of the reinforcement bars is in contact with a solid characterized by a highly basic environment pH 13
In this environment, the reinforcing iron creates a very thin layer (film) of ferrous hydroxide on the surface, insoluble very adherent, compact, non-porous, impenetrable, which does not allow oxygen and humidity to come into contact with the bars armor, preventing the formation of rust.
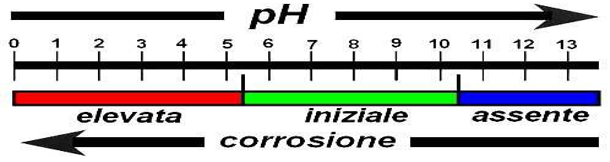
This effect, however, will continue to remain unchanged only if the pH of the conglomerate remains at very high values (constantly higher than pH 11).

In fact, the characteristics of the protective film are compromised if there is a decrease in pH (depassivation), that is when the concrete falls below the pH 11 threshold value
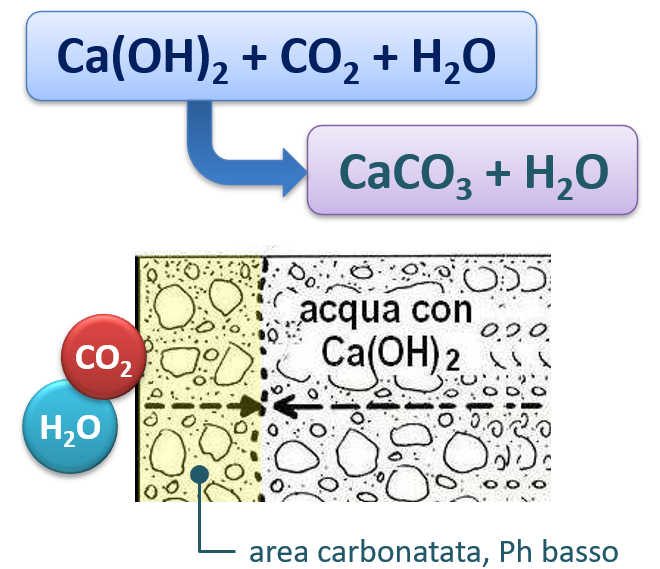
Carbonation is one of the most common causes of the lowering of the pH in the cement paste.
Carbonation: corrosion antechamber
By virtue of a spontaneous chemical reaction, in the presence of humidity, the calcium hydroxide (calcium hydroxide, Ca (OH)2) found in the cement paste combines with carbon dioxide, forming calcium carbonate (CaCO3)
Reinforcement corrosion: the quintessence of concrete degradation
What influences the trigger and the development of carbonation:
- CO2 permeability of concrete
- Exposure environment and in particular frequent alternations of dry and wet conditions
- Surface protection of the product (anti-carbonation treatments)
- Impermeability or not of the conglomerate
- discontinuity of the matrix (cracks)
- porosity of the matrix
The corrosion process leads to the formation of complex oxides, more voluminous than the non-oxidized metal alloy.
The increase in volume, in some cases, can even exceed 500%, frequently causing the destruction of the concrete cover